The world continues to be amidst a serious pandemic. While over 50 Covid-19 vaccine candidates are on trial, many countries have approved a number of them for emergency use to curb the fast-spreading Covid-19 virus.
Sri Lanka will receive its first consignment of vaccines on January 27. A stock of 600,000 vaccines will arrive from India allowing Sri Lanka to kick start its Covid-19 vaccination drive nearly a year after the first Covid-19 patient was reported in the country.
As a result in preparation and to ascertain how it should be conducted once Sri Lanka receives vaccine stocks health officials yesterday held a dry run at the Piliyandala MOH office, the Piliyandala district hospital, and the Colombo North Teaching hospital.
Before the trial, the Deputy Director-General of Health Services, Dr. Hemantha Herath said authorities hope to identify problems that can arise in the process.
“The trials will also be useful in deciding the number of vaccines that can be distributed within a certain timeframe,” he said. He also said health authorities in the country are prepared to distribute the vaccines as per the priority list as soon as Sri Lanka receives the vaccine shipment.
According to the State Minister of Urban Development, Dr. Nalaka Godahewa frontline workers, healthcare workers, and armed forces and police personnel are expected to receive the first jabs. Other vulnerable groups including elders and those with chronic illnesses will be next in line.
The State Minister said measures are under way to ensure all Sri Lankans also receive the vaccine. The WHO has also assured Sri Lanka that it would provide 20 percent of the required vaccines under its Covax program.
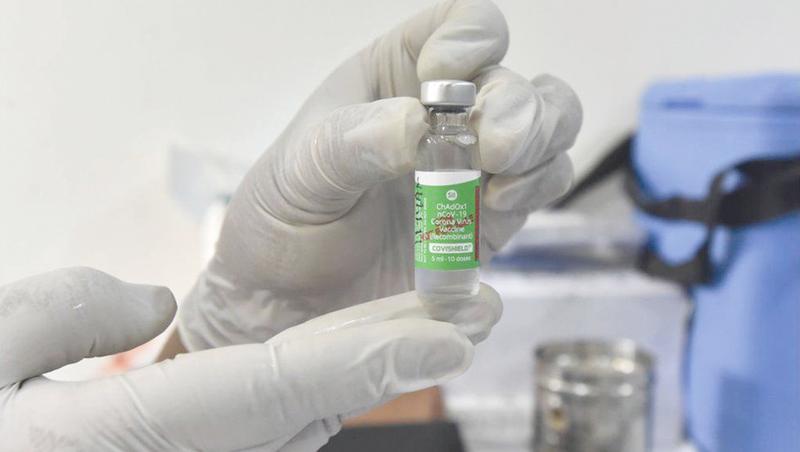
But the AstraZeneca/Oxford University Covishield vaccine, manufactured by Serum Institute of India will be the first vaccine to arrive on the shores of Sri Lanka according to the announcement made by President Gotabaya Rajapaksa even though Sri Lanka has also been holding discussions with Russia and China to obtain vaccines manufactured by them. Covishiled is a derivative of the AstraZeneca Oxford Vaccine developed under license in India.
While at the beginning of the Indian vaccine distribution program Sri Lanka had not provided regulatory clearances for the vaccine, the Government keen to commence the vaccine drive, granted regulatory approval on Friday, January 22 for the emergency use of Oxford-AstraZeneca Vaccine as confirmed by the State Minister of Production, Supply and Regulation of Pharmaceuticals, Prof. Channa Jayasumana.
Following this, the Indian High Commission in Sri Lanka stated that the Sri Lankan government conveyed that approval for the emergency use of Covidshield vaccines has been granted.
The Commission also said the Government authorities in India and Sri Lanka are now working towards the early delivery of these vaccines in Sri Lanka.
State Minister of Primary Health Care, Epidemics, and Covid Disease Control, Dr Sudharishini Fernandopulle had earlier assured Sri Lankans that the Covid vaccine drive in the country will commence in the early weeks of February. She said the vaccine will be provided free to prioritised target groups.
The State Minister also said Sri Lanka is hopeful of receiving doses of the PfizerBioNTech vaccine through the Covax facility of the World Health Organisation. “The prevailing infrastructure is being enhanced to facilitate the storage of both vaccines,” Dr. Fernandopulle said.
Though Sri Lanka now gears up to receive the Indian manufactured vaccine, a blazing fire that erupted at the main manufacturing plant of Vaccine-maker Serum Institute of India has now caused concern among the public about the effects it may have on vaccine production and supply.Five workers lost their lives in the blaze that fire erupted on Thursday, January 21. But the institute said the production or supplies of the Covid-19 vaccine remained unaffected.
“The fire incident will not affect Covishield supplies, but it has damaged the rotavirus and BCG vaccine manufacturing and storage facilities. It is a big financial loss for us, more than Rs 1,000 crore,” Serum Institute CEO Adar Poonawalla was quoted as saying.
According to him, the fire had taken place in another building and Covishield production and the stock were not affected. With India’s Foreign Minister Subrahmanyam Jaishankar assuring that Sri Lanka will be prioritized when Indian-produced Covid-19 vaccines are ready for export, Sri Lankans can allay all fears and remain hopeful that the vaccine will now roll out as promised by President Gotabaya Rajapaksa by next month.
